
PPT - LICKING THE SALT PROBLEM: Lowering sodium and maintaining quality in emulsions PowerPoint Presentation - ID:4998469

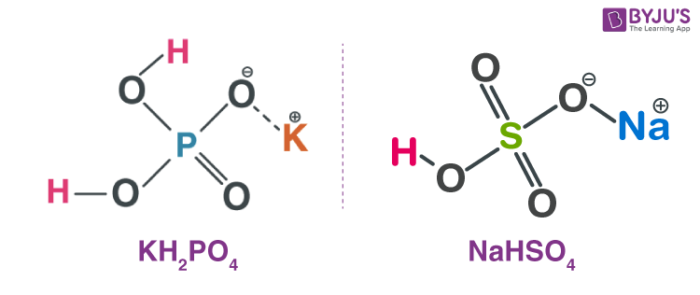
Double salts-definition-examples and properties in co-ordination chemistry. | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Salt Properties & Chemical Formula | What is Salt in Chemistry? - Video & Lesson Transcript | Study.com

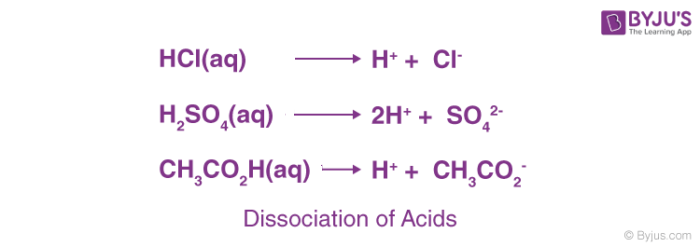
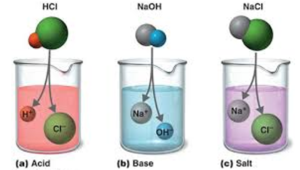
CHAPTER 8 : SALTS. Meaning and uses of Salts A salt is an ionic compound formed when the hydrogen ion, from an acid is replaced by a metal ion or an ammonium. -

Salt Definition, Formation, Examples, Properties and Uses | Some Common Salts Class 10 Chemistry - YouTube

Salt Properties & Chemical Formula | What is Salt in Chemistry? - Video & Lesson Transcript | Study.com









:max_bytes(150000):strip_icc()/GettyImages-148087170-3dd15f1a89b64644ba361e0bff5fbc60.jpg)




![10th STD Chemistry - Definition of Acids, Bases & Salts [ ICSE / CBSE / STATE BOARDS ] - YouTube 10th STD Chemistry - Definition of Acids, Bases & Salts [ ICSE / CBSE / STATE BOARDS ] - YouTube](https://i.ytimg.com/vi/HvZ71idT_n8/hqdefault.jpg)
:max_bytes(150000):strip_icc()/sodium-chloride-structure-artwork-160936423-589330f15f9b5874eea7ba04.jpg)